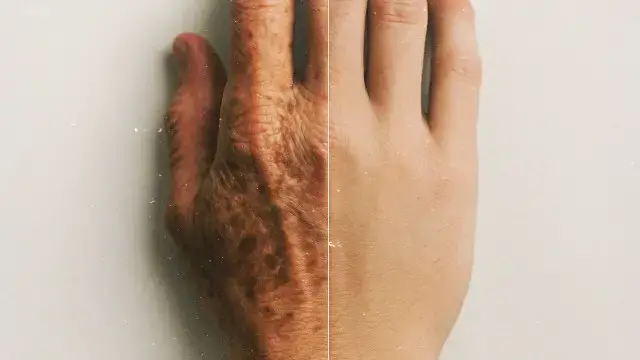
“Over the last 10 or 15 years, scientists have really started to understand the fundamental underlying biology of the aging process. And they broke this down into 12 hallmarks of aging.”
Show More Show Less View Video Transcript
0:00
I think when people hear about the idea of longevity science or longevity medicine
0:04
their brain instantly goes to trying to prevent death. It's billionaires taking hundreds of supplements a day trying to stay immortal
0:10
or people doing crazy exercise regimes and eating bizarre diets. It's when you eat, what you eat, how much you eat
0:16
And I think that this really is a distraction from the big goals of longevity science
0:21
We're not talking about immortality. This is about increasing your health span
0:25
increasing that period of life free from disease, free from pain, free from memory loss
0:29
that means we're going to need longevity medicine. And it sounds like this incredibly sci-fi, futuristic way
0:34
of making human beings live longer. But actually, we've already doubled what it means to be human
0:39
in life expectancy terms over the last 200 years. And the economic benefits of slowing down the ageing process could be huge
0:46
Economists have calculated that if we were able to slow down the ageing process and keep people healthier for just a single year
0:53
that would be worth $38 trillion. Three, eight, and then 12 zeros
0:57
By keeping people biologically younger, we can enjoy a longer period of healthy life
1:03
where we're active, where we're happy, where we can engage in our hobbies, we can play with our grandkids, our great-grandkids
1:07
This could be the greatest revolution in the history of medicine. I'm Dr. Andrew Steele. I'm a longevity scientist, writer and campaigner
1:14
and I wrote a book called Ageless, the new science of getting older without getting old
1:18
I actually started out as a physicist and I actually changed career to longevity science
1:31
because of a graph. It's a very simple graph. It's a graph of how old you are versus how likely you
1:36
are to die in that year. And it's pretty striking. I'm 39 years old and what that means is that my
1:43
risk of death this year is about one in a thousand. That means that if that were to continue for the
1:48
rest of my life, I'd live into my thousand and thirties on average. But unfortunately, of course
1:52
that doesn't happen. Our risk of death as human beings doubles about every eight years, so that
1:57
means there's an exponential increase. So if I'm lucky enough to live into my nineties, but unlucky
2:02
enough that we haven't made any advances in science or medicine in the intervening time, my odds of death will be about one in six. That's life and death at the roll of a dice
2:11
But for other animals around the animal kingdom, that can look very, very different. There are some animals, animals like giant tortoises or certain fish that live in the sea
2:18
there are certain salamanders that have a risk of death that doesn't double. Doesn't matter how long ago they were born, their risk of death stays flat
2:25
And so by this definition, these animals literally do not age, statistically speaking
2:30
There are two ways to look at this. You can either look at it as a human and think, this is this exponential, terrifying wall of mortality coming at me
2:36
Or you can look at it as a physicist trying to find the biggest problem in the world. and you can think well what is this fundamental ticking clock inside all of our biology that
2:45
massively increases our risk of cancer of heart disease of stroke of dementia of all these so-called
2:49
age-related diseases and if we could understand this ticking clock could we do something about it
2:55
in the last decade scientists have come up with various measures of what's called biological age
3:03
as distinct from chronological age so your chronological age is just how many candles
3:07
there are on your birthday cake, and obviously most of us are familiar with that. But the idea of biological age is to look inside your cells
3:13
look inside your body, and work out how old you are on a biological level
3:17
Now, we aren't perfect at doing this yet, but we do have a variety of different measures We can use blood tests we can use what are called epigenetic tests or we can do things that are far more sort of basic and functional How strong your grip is declines with age And by comparing the value of something like your grip strength to an average person of a given age
3:32
we can assign you a biological age value. And the ones that are getting the most buzz at the moment within the scientific community
3:38
but also all around the internet, are these epigenetic age tests. So the genome is your DNA, it's the instruction manual of life
3:46
And the epigenome is a layer of chemistry that sits on top of your genome. If you think of your DNA as that instruction manual
3:52
then the epigenome is the notes in the margin, it's the little sticky notes that have been stuck on the side
3:56
and they tell the cell which DNA to use at which particular time. And we know that there are changes to this epigenome as you get older
4:03
And so by measuring the changes in the epigenome, you can assign someone a biological age
4:08
I think the problem with these tests as applied to individuals is we don't know enough about exactly what they're telling us
4:13
We don't know what these individual changes in epigenetic marks mean. We know they're correlated with age
4:18
but what we don't know is if they're causally related and so what we need to do is more
4:22
experiments where we try and work out if we can intervene in these epigenetic and these biological
4:26
clocks. Over the last 10 or 15 years scientists have really started to understand the fundamental
4:33
underlying biology of the aging process and they broke this down into 12 hallmarks of aging
4:39
One of those hallmarks is the accumulation of senescent cells. Now senescent is just a biological
4:44
technical term for old. These are cells that accumulate in all of our bodies as the years go by
4:49
And scientists have noticed that these cells seem to drive a range of different diseases as we get
4:53
older. And so the idea was, what if we could remove these cells and leave the rest of the
4:58
cells of the body intact? Could that slow down or even partially reverse the aging process
5:04
And scientists identified drugs called senolytic drugs. These are drugs that kill those senescent
5:08
cells. And they tried them out in mice, and they do indeed effectively make the mice biologically
5:13
younger. Firstly, it makes them live a bit longer. It's a good thing if you're slowing down the aging
5:17
process, the basic thing you want to see. But it's not dragging out that period of frailty at the end
5:21
of life. They get less cancer, they get less heart disease, they get fewer cataracts. And what this
5:26
shows us is that by targeting the fundamental processes of aging, by identifying something like
5:30
senescent cells that drives a whole range of age-related problems, we can hit many, perhaps even
5:34
all of those hallmarks. One of the most exciting ideas in longevity science at the moment is what's
5:40
called cellular reprogramming. I sometimes describe this as a treatment that has fallen
5:45
through a wormhole from the future. This is the idea that we can reset the biological clock inside
5:49
of our cells. And the idea first came about in the mid-2000s. There was a scientist called Shinya
5:55
Yamanaka who was trying to find out how to turn regular adult body cells all the way back to the
6:00
very beginning of their biological existence, the time when they were an embryo. Now he was interested
6:04
in this from the point of view of creating stem cells, a cell that can create any other kind of
6:09
cell in the body, which we might be able to use for tissue repair in future. But scientists also
6:13
noticed, as well as turning back the developmental clock on these cells, it also turns back the
6:18
aging clock. Cells that are given these four Yamanaka factors actually are biologically younger
6:24
than cells that haven't had the treatment. And so what scientists decided to do was insert these
6:28
Yamanaka factor genes into mice. Now if you do this in a naive way, so those genes are active all the
6:33
time, it's actually very bad news for the mice unfortunately, because these stem cells, although
6:37
they're very powerful in terms of what kind of cell they can become, they are useless at being
6:41
a liver cell or being a heart cell, and so the mice very quickly died of organ failure
6:46
But if you activate these genes only transiently and the way that scientists did it the first time successfully was essentially to activate them at weekends They found that this was enough to turn back the biological clock in those cells but without turning back the developmental clock
6:58
and turning them into these stem cells. Now, the real challenge is this is a gene therapy treatment
7:03
It involves delivering four different genes to every single cell in your body. And so the question is
7:07
is can we, with our puny 2020s biotechnology, make this into a viable treatment, a pill even
7:12
that we can actually use in human beings? And that's what's led various different billionaires from the Bay Area to invest huge, huge amounts of money in this
7:19
Altos Labs is the biggest so-called startup in this space. And I wouldn't really call it a startup because it's got funding of $3 billion from amongst other people Jeff Bezos
7:28
Now, I'm very excited about this because I think $3 billion is enough to have a good go and see if we can turn this into a viable human treatment
7:35
My only concern is that epigenetics is only one of those hallmarks of aging
7:40
For example, resetting the epigenetic clock can't help with mutations in our DNA that accumulate throughout the course of our lives
7:46
It also can't help with anything that happens outside of cells. For example, damage to the proteins that are the scaffolding of our body, things like collagen that make up our skin and our bones
7:54
And so it might be the case that we solve aging inside our individual cells, but we leave other parts of the aging process intact
8:03
Probably the quickest short-term wins in longevity science are going to be repurposed existing drugs
8:09
And the reason for this is because we've spent many, many years developing these drugs. We understand how they work in humans
8:14
We understand a bit about their safety profile. And because these molecules already exist, we've just tried them out in mice, in various
8:20
organisms in the lab, and found that a subset of them do indeed slow down the aging process
8:26
The first trial of a longevity drug that was proposed in humans was for a drug called metformin
8:31
which is a pre-existing drug that we prescribe actually for diabetes in this case, and has
8:35
some indications that it might slow down the aging process in people. The proposal in the trial is to observe a number of different diseases
8:42
So we see if the people who are taking the real metformin, do they get cancer later? Do they get dementia later
8:46
Do they get less heart disease? Do they die later than people in the control group? And if you follow these people for three to five years
8:52
that should give us enough data to understand whether metformin slows down the aging process
8:58
I think one of the ones that's got the most buzz around it at the moment is a drug called rapamycin
9:02
It was first isolated in bacteria from a soil sample from Easter Island
9:06
and it was discovered to be antifungal. It could stop fungal cells from growing
9:10
But when the scientists started playing around with it in the lab, they realized it didn't just stop fungal cells from growing
9:15
It also stopped human cells. The way that repamycin works is it targets a fundamental
9:21
central component of cellular metabolism. It starts this process called autophagy, which is Greek for self-eating, autophagy
9:28
And that means it consumes old damaged proteins and then recycles them into fresh, new ones
9:33
And in 2009, we found out for the first time that by giving it to mice late in life
9:38
you could actually extend their remaining lifespan. They live by 10 or 15% longer. And this was a really incredible result
9:43
This was the first time a drug had been shown to slow down aging in mammals. And we've now tried it in loads of different contexts
9:49
in loads of different animals, in loads of different organisms, at loads of different times in life. And that's fantastic news potentially for us humans
9:55
because not all of us, unfortunately, can start taking a drug from birth because most of us were born quite a long time ago
10:00
Because we understand a lot about these drugs, it means we could start doing a human trial relatively soon
10:04
And what I mean by that is that we could have the first longevity drug in the next 5 or
10:08
10 years if we give this science sufficient funding. We could imagine doing longevity gene edits in human beings perhaps not in the next 5 years but I think it would be foolish to bet against it happening in the next 20 years for example And again that in plenty of time for most people watching this video
10:24
I find it really fascinating that I get so many ethical questions because of this work
10:29
If I was instead a cancer researcher and I was telling people we had this amazing new breakthrough that was going to solve childhood leukaemia, I would not get people standing
10:36
up at the end of a talk and asking me, Andrew, aren't all these kids going to be bored with
10:40
that extra life that you've got them? Aren't we going to have overpopulation because we're going to have all of these cancer survivors
10:44
clogging up the planet and using resources and destroying the environment? And yet, when you start talking about longevity, suddenly all of these questions come out of the woodwork
10:52
It's as though we place it in a completely separate moral, ethical category to any other kind of medicine
10:57
And what I believe is that this longevity science is just an extension of modern medicine
11:03
At the moment, ageing is the leading cause of death globally. Over 100,000 people die every single day of cancer, of dementia, of the increased risk of infectious disease that comes along with growing older
11:14
And if we can tamp down on that increase of risk of death with time that comes along with being a human being
11:19
it could allow us not to just develop treatments for individual diseases, but drugs that can prevent multiple different diseases from ever arising in the first place
11:27
But it gets shockingly little funding. In the United States, government funding of this kind of science is just over $1 per American
11:35
which is absolutely remarkable given that you think about aging being the cause of cancer and
11:39
dementia and increased infection risk and so on and so on. If you add together all those causes of
11:44
death, aging causes 85% of deaths in the US. We really need a giant project on the scale of the
11:50
Human Genome Project to understand how people age because there are so many different things
11:56
happening in all of our biology. We need to measure not just your DNA but how the expression
12:00
of your genes changes, how your cells change, how the proteins in your blood and between your cells
12:04
change. We need to monitor all of these things in order to try and feed them into a giant computer
12:09
model and create what we'd call a systems biology model of a human being. There are already people
12:15
talking about the idea that AI could cure all disease in the next five or ten years. And I think
12:19
the thing this really misses is that AI is only as good as the data that you train it on. And I think
12:24
a great example of this is something called AlphaFold, which is an AI that can predict how a protein is
12:29
folded purely from its sequence. And this was a huge scientific breakthrough, but it was only
12:34
made possible because for decades scientists have been measuring the structures of proteins and
12:39
depositing them in a centralized database called the Protein Databank. Now it's estimated that this
12:44
database would cost 21 billion dollars to rebuild if we were to suddenly accidentally delete it
12:48
today. And that means it's a hugely hugely valuable resource. It contains 160,000 protein structures
12:54
and that was enough protein structure data to train an AI that can then predict the structure
12:58
out of a protein it's never seen before. So if we want an AI that can predict what's going on inside of human biology
13:03
we're going to need to invest a similar amount of money. But with a 38 trillion potential economic benefit
13:08
and actually even more than that, if we can extend life by more than a year, I think the payoff would be absolutely worth it
13:14
This is just a hugely exciting time to be alive. Maybe for a little bit longer than we thought
#Aging & Geriatrics