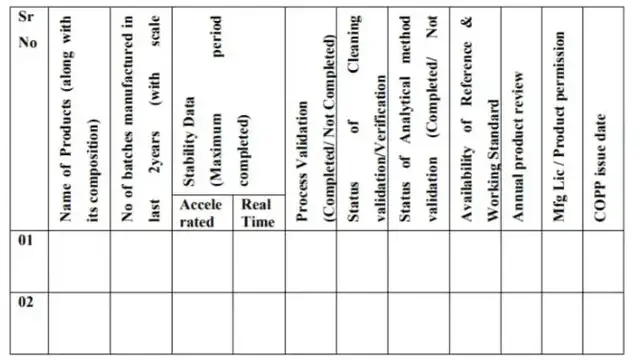
WHO-GMP Document Check List for Visitors
Apr 5, 2023
WHO-GMP Document Check List is very effective documents for visitors.
This video provides an overview of the WHO-GMP Document Check List for Visitors, a critical tool for ensuring compliance with the WHO Good Manufacturing Practices guidelines. Viewers will learn about the essential documents that should be reviewed during a facility visit, including Standard Operating Procedures, batch records, and quality control documents.
WHO-GMP Document Check List, Good Manufacturing Practices, Compliance, Facility Visit, Standard Operating Procedures, Batch Records, Quality Control, Pharmaceutical Manufacturing, FDA, EMA, Pharmaceutical Industry.
Show More Show Less 