Understanding Deviation: Types, Impact, and Strategies in Pharmaceutical Manufacturing
Apr 16, 2023
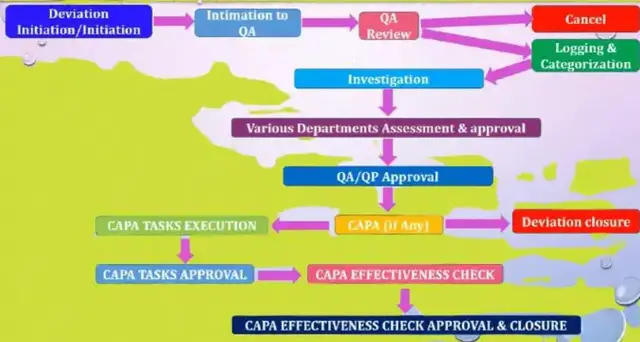
This article delves into the world of deviations in pharmaceuticals, starting with an introduction to what they are and why they matter. It then explores the common types of deviations that occur during the manufacturing process and how they can impact product quality and safety. Finally, readers will learn about strategies for managing and preventing deviations in order to ensure the highest level of quality control possible. Whether you're a seasoned professional or just starting out in the industry, this comprehensive guide is a must-read for anyone looking to stay ahead of the curve in pharmaceutical manufacturing.
In this video, we will explore deviations in pharmaceutical manufacturing, their impact, and strategies to minimize their occurrence. We will cover different types of deviations and their classification, causes of deviations, and how to investigate and document deviations. We will also discuss the impact of deviations on pharmaceutical products' quality, safety, and efficacy and regulatory compliance. Finally, we will suggest strategies to prevent deviations and corrective actions to mitigate their effects.
Pharmaceutical manufacturing, Deviations, Types, Impact, Strategies, Quality, Safety, Efficacy, Regulatory compliance, Investigation, Documentation, Corrective actions, Preventive measures, GMP, FDA, Compliance, Manufacturing process, Risk management, Root cause analysis, SOP, CAPA.
Show More Show Less 