GxP in Pharmaceuticals industries (FDA guidelines)
Apr 16, 2023
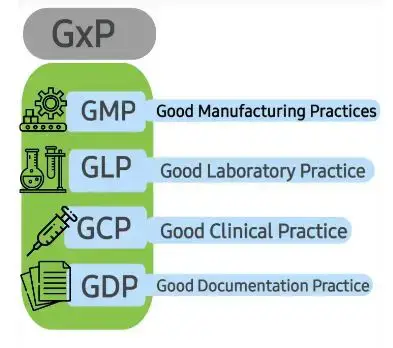
GxP in Pharmaceuticals industries (FDA guidelines). In this video, we will discuss GxP regulations in the pharmaceutical industry, specifically the FDA guidelines. GxP refers to a set of quality guidelines that ensure the safety, efficacy, and consistency of pharmaceutical products. We will cover the key concepts of GxP, including Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and Good Clinical Practices (GCP). We will also discuss the importance of complying with these regulations and the consequences of non-compliance.
GxP regulations in the pharmaceutical industry
FDA guidelines for GxP
Good Manufacturing Practices (GMP)
Good Laboratory Practices (GLP)
Good Clinical Practices (GCP)
pharmaceutical industry compliance
consequences of non-compliance in the pharmaceutical industry
pharmaceutical industry safety and efficacy
pharmaceutical product consistency
quality guidelines in the pharmaceutical industry.
Show More Show Less 